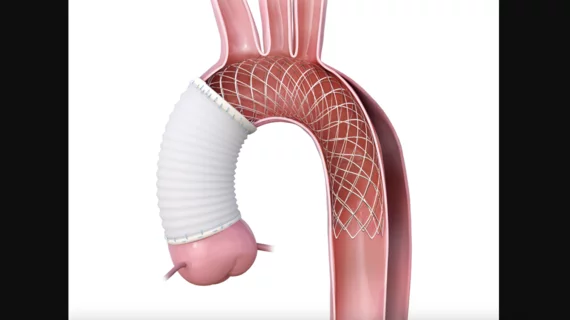
The Atlanta-based company said AMDS is the first aortic arch remodeling device to treat acute DeBakey Type I aortic dissections.
An HDE designation allows for commercial distribution of the device in the U.S. before Artivion’s anticipated FDA premarket approval. Under the designation, AMDS will be available as a treatment for acute DeBakey Type I dissections in the presence of malperfusion. Once approved, the device is expected to cover all acute DeBakey Type I dissections with and without malperfusion.