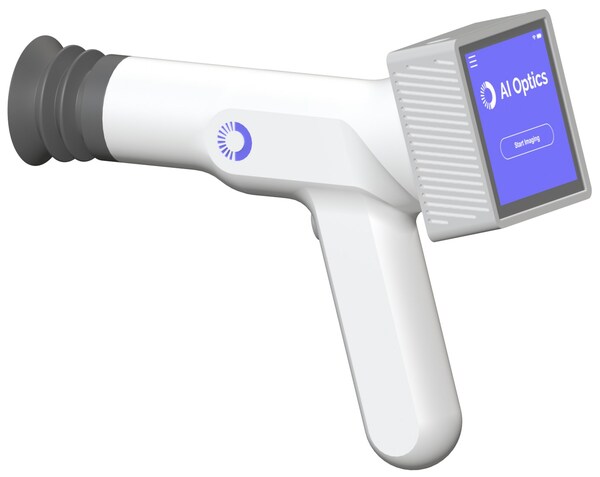
Over one billion people are at risk for retinal diseases, yet access to screening remains limited due to specialist shortages, cost, and convenience barriers. The Sentinel Camera helps tackle these challenges by enabling point-of-care retinal imaging, eliminating the need for some patients to visit an eye specialist’s office. It is intentionally designed to be easy to use and support non-dilated imaging, while being both affordable and portable. The design was also developed to meet the needs of general healthcare providers, including primary care, optometry, retail health clinics, and home health settings, ensuring accessibility and practicality in these environments.
Collaborating with NYU Langone Health
AI Optics is strategically collaborating with NYU Langone Health to advance the accessibility and implementation of retinal screening technology. This partnership aligns with AI Optics’ mission to address the global challenge of preventable blindness by providing innovative tools that meet the needs of healthcare providers and patients. The FDA clearance of the Sentinel Camera marks a pivotal step in this journey, enabling the integration of high-quality, portable imaging into diverse healthcare environments.