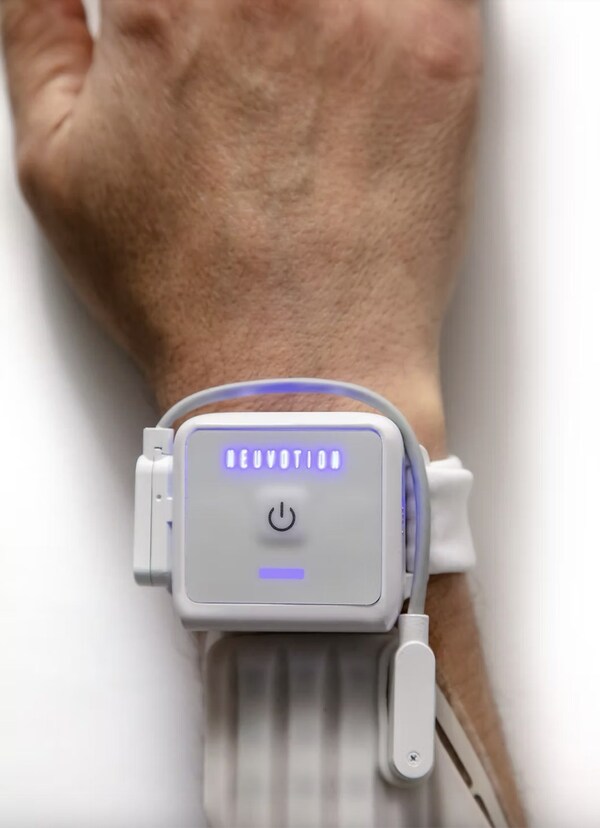
“At Neuvotion we are developing highly innovative technologies built on two decades of scientific research yielding effective and easy-to-use products,” said Chad Bouton, Neuvotion’s founder and CEO. “We are extremely excited about NeuStim™, our first product, which we believe will be a game-changer in the neuromodulation, brain-computer interface, and neurorehabilitation markets.”
“We have been very impressed with Professor Bouton’s groundbreaking foundational research and Neuvotion’s highly innovative approach,” said Michael Spigel, PT, MHA, President & CEO of Good Shepherd Rehabilitation. “We look forward to continuing as a clinical research partner with Neuvotion. We feel that NeuStim™ is truly revolutionary and will help produce improved outcomes in stroke and spinal cord injury rehabilitation.”