The trial will evaluate the safety and effectiveness of the eShunt System versus the current standard of care, the ventriculo-peritoneal (VP) shunt, in draining accumulated cerebrospinal fluid from the brain in elderly patients. The results of the STRIDE trial will serve as the basis for CereVasc’s anticipated submission to regulatory agencies for approval to market the eShunt System.
“Treatment is essential for patients with NPH, as this progressive, life-threatening form of communicating hydrocephalus can cause symptoms including cognitive dysfunction, gait disturbance and urinary incontinence,” said Pedro Lylyk, M.D., CEO of ENERI and Clinica la Sagrada Familia in Buenos Aires and co-lead investigator of the STRIDE trial. “Positive findings from the STRIDE trial will broaden access to NPH treatment in Argentina, as more patients would likely be eligible for a minimally invasive procedure than for open brain surgery.”
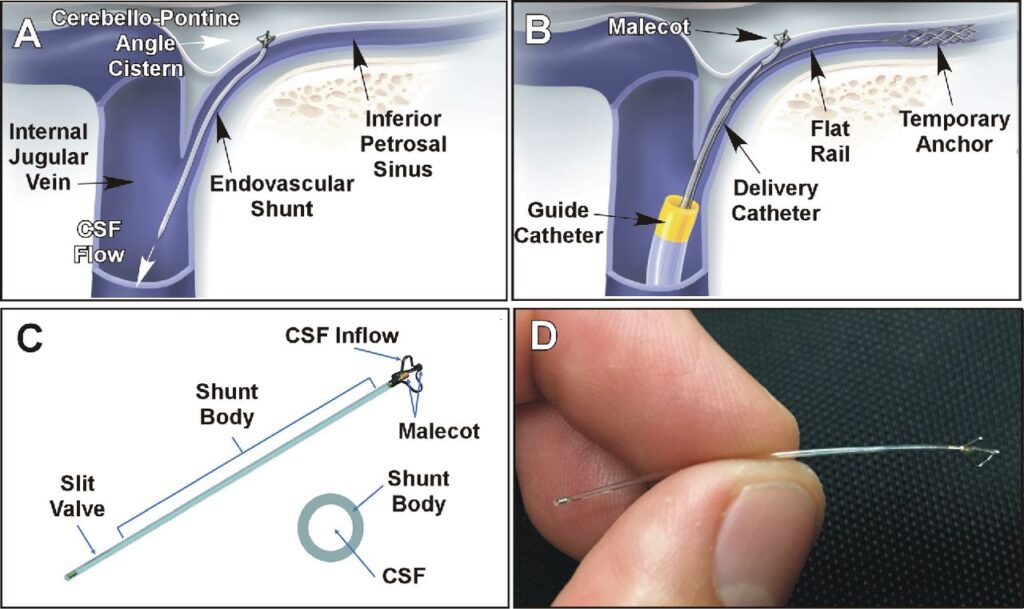
The eShunt System is the only minimally invasive, endovascular shunt and the first new treatment option developed for NPH since the VP shunt was introduced more than 60 years ago.