According to the FDA, it granted 510(k) clearance for the system on March 25, about two months post-submission (Jan. 27).
The database included the RESTORE ATK (above the knee) trial for Bolt IVL in treating stenotic lesions. According to the trial’s information provided on ClinicalTrials.gov, the study of 97 subjects was completed in July of 2024. That conclusion came about six months before Boston Scientific agreed to acquire Bolt Medical and its IVL technology. Boston Scientific initially developed the concept for the Bolt IVL system, helping establish Bolt Medical in 2019. As a strategic investor, the Marlborough, Massachusetts–based company already has an equity stake of approximately 26%.
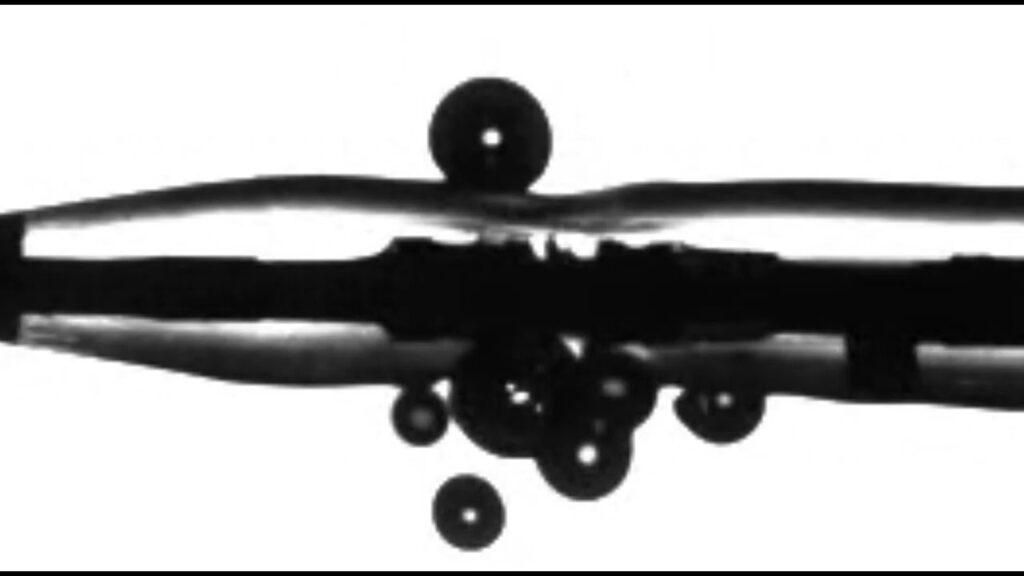
Bolt IVL was designed to enhance percutaneous transluminal angioplasty by utilizing delivery of IVL to disrupt calcium prior to full balloon dilatation at low pressures.
The advanced laser-based platform treats of coronary and peripheral artery disease. Its novel application of lithotripsy fractures calcium by creating acoustic pressure waves inside a balloon catheter. The system also includes visible, directional emitters for consistent energy delivery in the treatment of calcified lesions.