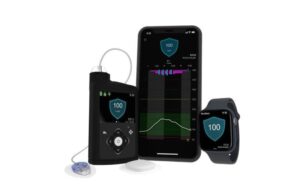
Approval provides more options for users of the automated insulin delivery (AID) system, which can also work with the Guardian 4 sensor.
Simplera Sync, a disposable, all-in-one sensor requires no fingersticks or overtape. It works with SmartGuard technology and features a simple, two-step insertion process.
Medtronic initially won FDA approval in August for its next-generation Simplera CGM. The Simplera platform includes the Simplera CGM for use with the InPen smart insulin pen and Simplera Sync specifically for integration with the MiniMed 780G. The medtech giant picked up CE mark for Simplera Sync with MiniMed 780G in January 2024.