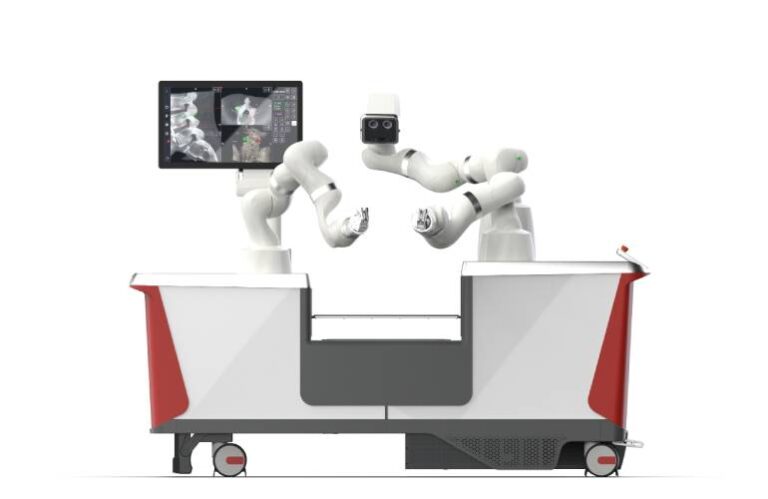
Bern, Switzerland-based LEM Surgical has its U.S. offices and a surgical demonstration suite for its robot in Tampa, Florida. It designed Dynamis as an integrated, navigation-based robotic platform. It enhances accuracy and control during spine surgeries. Clearance provides U.S. spine surgeons with the next-generation technology that combines real-time imaging, dynamic guidance and adaptable instrumentation compatibility within a single, versatile platform.
Dynamis features three robotic arms, with two for surgical guidance and one for optical navigation. They consolidate into a single cart that fits partially beneath the surgical table. Unlike currently available systems, LEM Surgical says its system supports a wide range of surgical instruments through unique, adjustable end-effectors and intraoperative qualification. This enhances compatibility and hospital workflow efficiency.