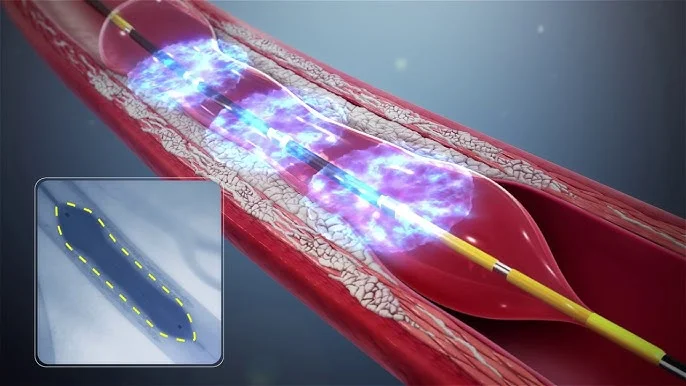
FastWave Medical has received Institutional Review Board (IRB) approval to initiate a coronary feasibility study for its innovative Sola™ laser intravascular lithotripsy (L-IVL) system. This marks a major milestone for the company as it prepares for a pivotal U.S. trial in collaboration with Clinical Accelerator. The study aims to evaluate the safety and effectiveness of Sola™ in treating calcified coronary artery disease—a condition that often complicates interventional cardiology procedures and affects patient outcomes.
The Sola™ L-IVL platform is a next-generation system that combines a laser-based energy source with a rupture-resistant balloon catheter. Unlike traditional IVL devices, Sola™ produces precise, circumferential sonic pressure waves that allow physicians to effectively modify hardened calcium deposits with improved consistency and control. This technology is designed to enhance procedural safety and reduce complications in complex coronary interventions. Physicians and clinical experts have highlighted its user-friendly design and the potential to significantly improve treatment precision for calcified arterial lesions.