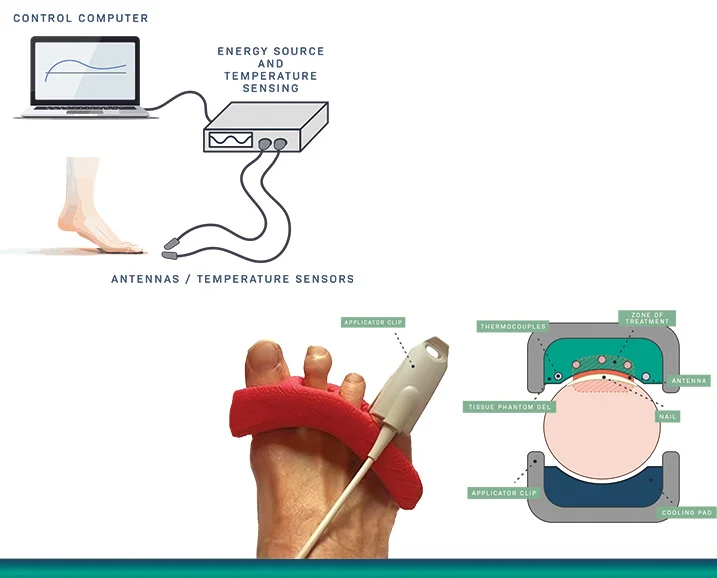
BiaCure Therapies has received a $2.6 million Small Business Innovation Research (SBIR) Phase I grant from the National Institutes of Health (NIH) to develop its novel high-frequency energy (HFE) therapy system, BiaPulse™, for the treatment of onychomycosis—a persistent fungal infection of the nails. The condition, which affects over 35 million Americans, is notoriously difficult to treat with existing oral medications due to poor efficacy and significant side effects. BiaPulse™ is a non-invasive therapeutic device designed to deliver targeted high-frequency energy directly through the nail, offering a patient-friendly alternative that eliminates the need for systemic drug exposure.
The innovation behind BiaPulse™ lies in its ability to deliver short-course, high-frequency energy via a specialized applicator that penetrates the nail bed without causing damage or discomfort. This energy disrupts the fungal environment, aiming to eradicate the infection at its source. Unlike traditional treatments that require long-term oral medication or repeated topical application with limited success, BiaPulse™ promises a faster, safer, and more effective solution. The technology is being developed in collaboration with leading dermatology and podiatry experts to ensure clinical efficacy, with trials measuring fungal eradication and patient outcomes.