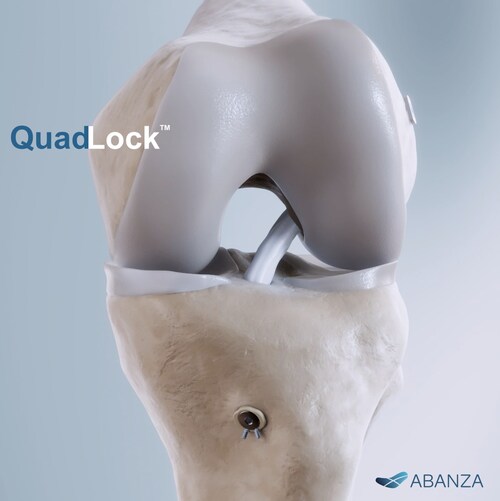
QuadLock™ is designed to securely fixate sutures and tapes, helping surgeons fine-tune graft tension and maintain stability across several graft configurations commonly used in ACL procedures, including quadriceps tendon, quadrupled semitendinosus/gracilis, and bone–patellar tendon–bone (BTB).
In biomechanical testing under high-demand cyclic loading, QuadLock™ demonstrated market-leading control of cyclic displacement (less than 0.5 mm)—a 500%+ improvement compared to the 3–6 mm reported for conventional fixation methods such as cortical buttons and interference screws. QuadLock™ also achieved a pullout strength of >1,000 N, combining high fixation strength with minimal displacement. Avoiding loss of tension under repeated loading is critical in early recovery, when fixation stability directly influences the restoration of functional joint stability.