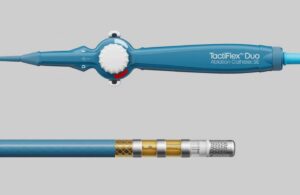
Abbott SVP Uri Yaron said on LinkedIn that the FDA granted the breakthrough nod for the TactiFlex Duo Sensor-Enabled catheter for the treatment of ventricular tachycardia (VT) using pulsed field ablation (PFA). The catheter features a dual-energy modality for treating VT using pulsed field ablation (PFA).
“To qualify for this breakthrough designation, products must address an unmet need and show it has the potential to provide more effective treatment of life-threatening diseases,” Yaron wrote. “This is at the heart of what we do at Abbott. This milestone represents years of dedication in developing innovations that support physicians as they treat patients with complex arrhythmias.