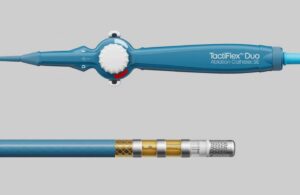
Following approval, the company also reported the first commercial cases using the catheter in Europe, completed this week. Dr. Isabel Deisenhofer, head of the department of Electrophysiology at the German Heart Center Munich, became one of the first to use TactiFlex Duo after its approval.
TactiFlex Duo represents Abbott’s latest advancement within its pulsed field ablation (PFA) portfolio. The medtech giant designed the system to deliver tailored therapy lesions in two ways. Its radiofrequency energy capabilities use heat to destroy tissue responsible for erratic heart signals. The pulsed field function uses high-energy electrical pulses to destroy the cells causing abnormal heart rhythms. This can reduce the risk of damaging adjacent tissue in patients with complex disease or anatomy.