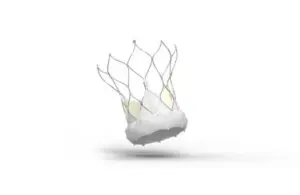
The expanded indication enables treatment for symptomatic severe aortic stenosis in those at low or intermediate risk for open-heart surgery. Abbott previously received CE mark for the Navitor transcatheter aortic valve implantation (TAVI) system in 2021. That indication covered symptomatic severe aortic stenosis in those at high or extreme surgical risk.
With the new approval, Abbott said it can offer Navitor in Europe for patients across all surgical risk categories. This significantly expands the treatable population for the device. Navitor currently has U.S. approval to treat symptomatic severe aortic stenosis in those at high or extreme risk for open-heart surgery. Abbott launched the latest generation of the valve last year.