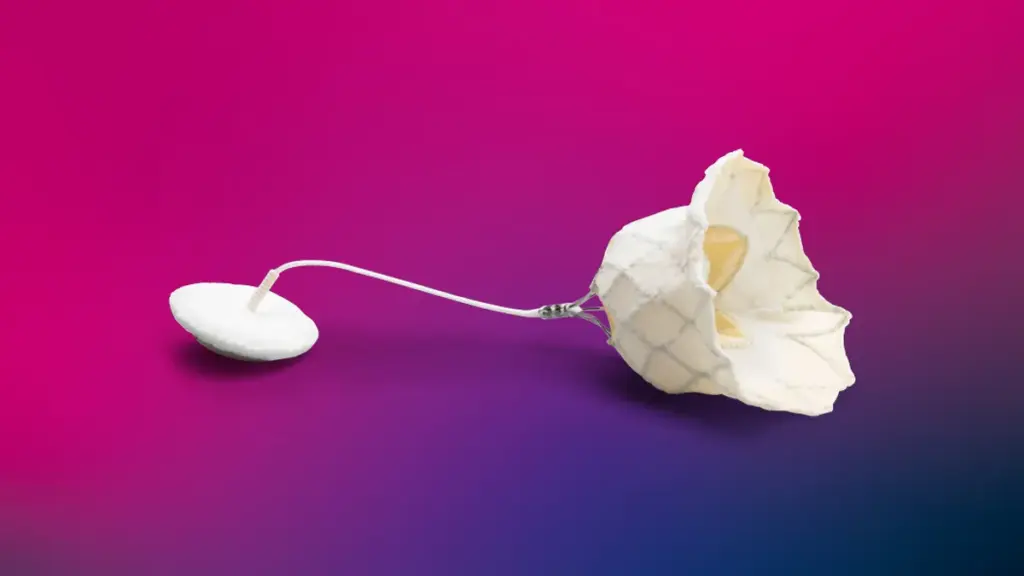
Abbott said Tuesday it received Food and Drug Administration approval for the Tendyne transcatheter mitral valve replacement system to treat calcium buildup in the ring that supports the heart valve.
The device is available for patients with severe mitral annular calcification who are not candidates for open heart surgery or transcatheter mitral valve repair.
Abbott’s MitraClip system for mitral valve repair competes with Edwards Lifesciences’ Pascal repair device. The rivals are now set to compete in mitral valve replacement: Edwards won Europe’s CE mark last month for the Sapien M3 transfemoral system and expects U.S. approval in 2026.