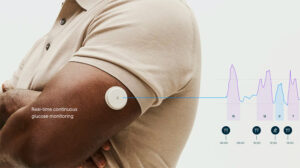
The clearance came on May 29, according to a listing on the FDA’s website. An Abbott spokesperson said the company was aware of the clearance and would provide more details later.
FDA clearance for Abbott’s Lingo comes more than two months after Dexcom won clearance for its Stelo over-the-counter CGM. Officials at both companies have expressed excitement over selling CMGs to athletes and others who may not have diabetes but find benefits from tracking their blood glucose.