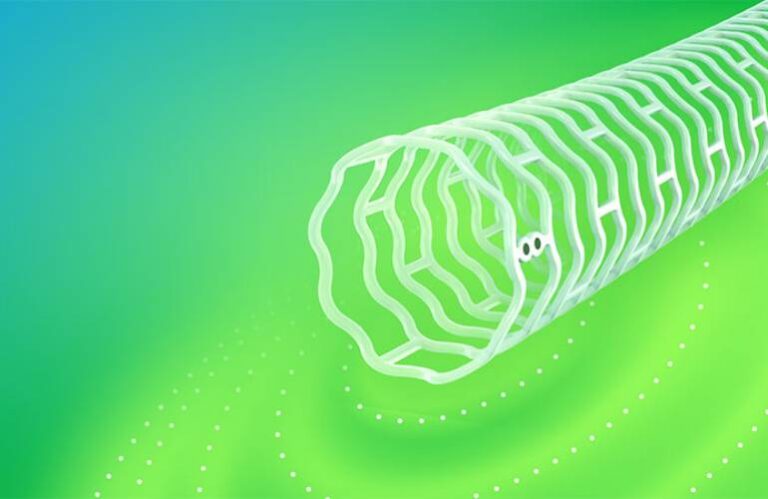
The Esprit BTK system is implanted through a catheter-based procedure and is made of material similar to dissolving sutures. The device supports the artery and delivers everolimus to aid vessel healing for about three years before dissolving. Abbott said the approach addresses limitations of balloon angioplasty, the current standard of care, which often results in re-narrowed vessels and repeat procedures.
Clinical results from the LIFE-BTK trial showed the device reduced repeat procedures by 48% compared to balloon angioplasty at two years.