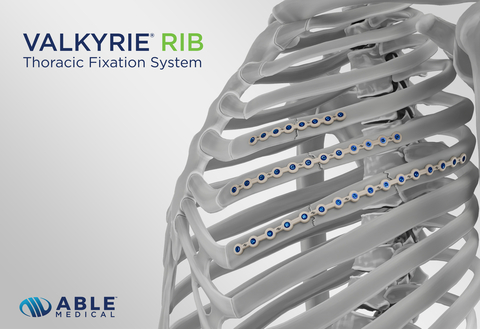
MARQUETTE, Mich.–(BUSINESS WIRE)– Able Medical Devices has received U.S. FDA 510(k) clearance for its Valkyrie RIB System further enhancing its cardiothoracic and trauma portfolio. The novel single-use, PEEK device is an extension of Able’s Valkyrie Thoracic Fixation System and includes indications for the stabilization and fixation of fractures in the chest wall, reconstructive surgical procedures, trauma, and planned osteotomies.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240125747360/en/
“The Valkyrie RIB Thoracic Fixation System creates an adaptive fit to the bone, promoting closer contact with the underlying rib without the need for plate bending tools,” said Wade DePas, Senior Director of RAQA and R&D. “Supported by material science, this novel system is rooted in clinical history and prioritizes adaptability over the conventional norms of rigidity.”
Focusing on material science, the PEEK-based Valkyrie RIB System combines durable fixation with flexibility to help minimize patient stress shielding and conforms to meet various patient anatomies. The double-lead screws insert into the bone faster, increase bone purchase, and the “zero-chance” cross threading design reduces concern of screw back out.