The Palm Beach Gardens, Florida-based company said the clearance followed a series of safety tests and modeling studies that evaluated magnetic field interactions, heating effects, and image artifacts. The implants met ASTM standards for MRI compatibility under the approved scanning parameters.
“The ability to confidently undergo MRI scans is an important factor for both patients and surgeons when selecting spinal implants,” Accelus President and CEO Kevin McGann said in a news release. “This additional clearance reinforces our commitment to offering safe, high-quality solutions that address the real-world needs of the spine surgery community.”
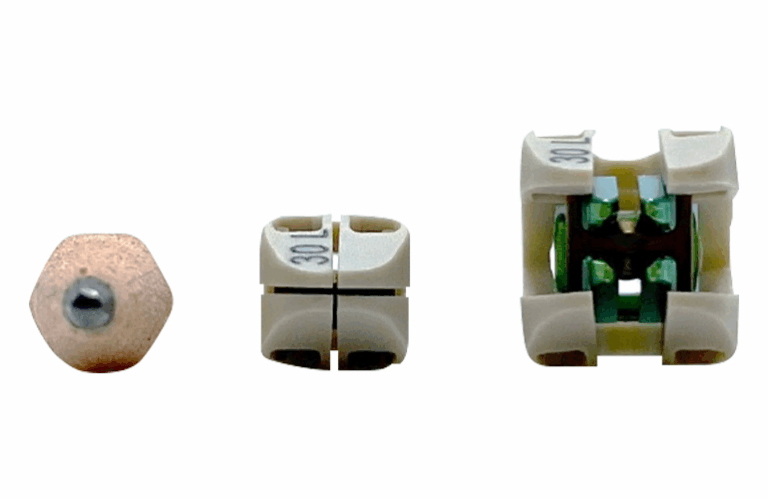
Accelus designed the FlareHawk system with a PEEK shell and internal titanium components. It allows for a small insertion profile to minimize tissue disruption, followed by multidirectional expansion to restore foraminal height and alignment while aiming to reduce the risk of implant subsidence.