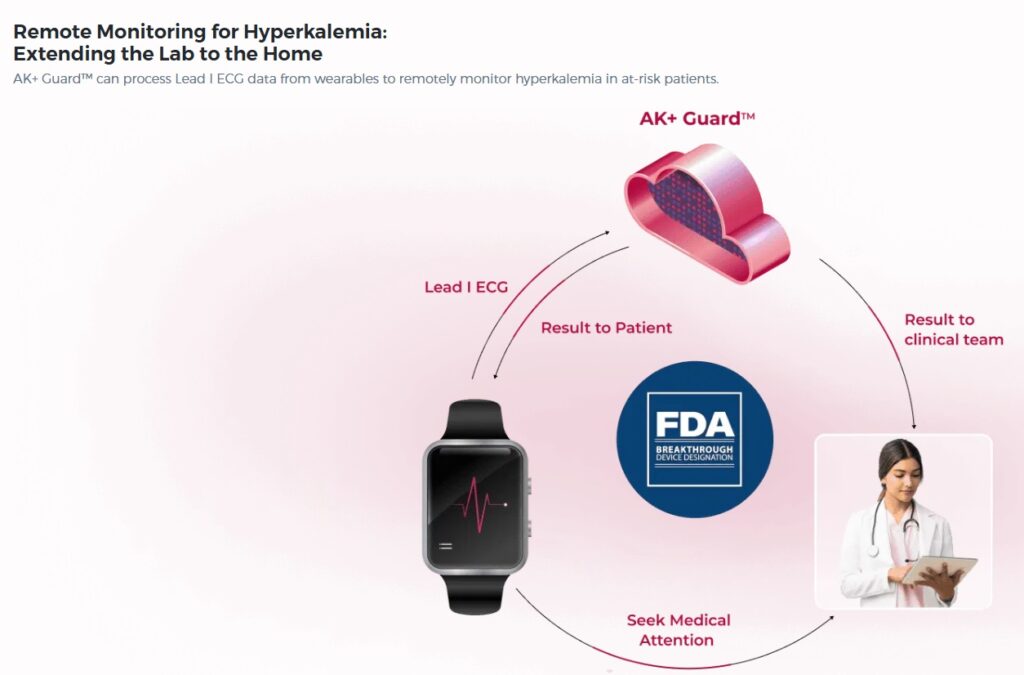
The AK+ Guard software uses Lead I ECG data to alert patients and clinicians of moderate to severe episodes of hyperkalemia. These episodes of excess potassium in the blood can lead to sudden cardiac arrest, according to a news release. In addition to breakthrough designation, the FDA accepted the software into its Total Product Life Cycle Advisory Program (TAP).
AccurKardia designed AK+ Guard to work with a wide range of FDA-cleared consumer and clinical wearables. These wearables, such as smartwatches, currently capture Lead I ECG data. This combination could enable hyperkalemia monitoring outside of the clinic and earlier intervention for high-risk populations. That includes those with end stage renal disease, chronic kidney disease (CKD) and other risk factors.