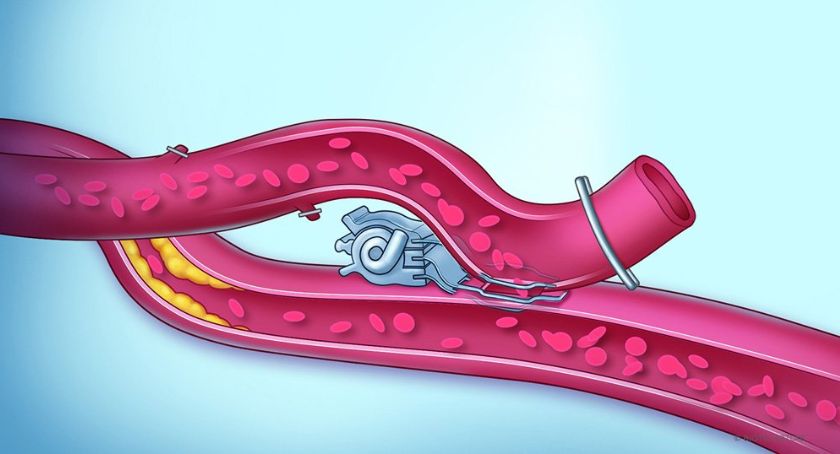
The Laguna Hills, California–based company based its vCLAS cryoablation catheter on its proprietary ultra-low cryoablation technology (ULCT). It aims to address the challenges of ventricular tachycardia (VT) ablations, comprising part of the Adagio VT cryoablation system. The system’s features make it time- and effort-efficient across the range of purely endocardial ablation strategies in patients with multiple VT etiologies.
Adagio currently has vCLAS under evaluation in an FDA investigational device exemption (IDE) study called FULCRUM-VT. Earlier this year, the company executed a restructuring effort to prioritize FULCRUM-VT. With layoffs included, the restructuring also centered focus on a new product design optimization program.
The FDA breakthrough nod provides priority review and interactive communication during the premarket review phase.