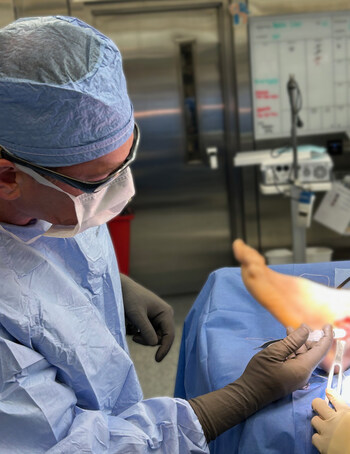
VersaCoat Flowable Hydrogel, developed from Alafair’s proprietary VersaCore™ Hydrogel Technology, uses the same collagen-free hyaluronic acid and alginate formulation as VersaWrap® Hydrogel Sheet (VersaWrap® Tendon Protector, VersaWrap® Nerve Protector). Hyaluronic acid is essential during the early stages of soft tissue healing, supporting hydration and reducing inflammation. VersaCore’s alginate bioresorbs to progressively expose bioavailable hyaluronic acid throughout the healing process. Both polymers fully bioresorb without leaving residual material, allowing tissue to return to its pre-injury state.
VersaCoat Flowable Hydrogel is designed to provide a conformable, bioresorbable, and tissue-adherent matrix to allow gliding and to limit postoperative soft tissue tethering. The novel syringe delivery allows precise placement onto delicate and hard-to-reach anatomy, expanding the benefits of the company’s clinically proven VersaCore Hydrogel Technology into anatomically complex surgical sites.