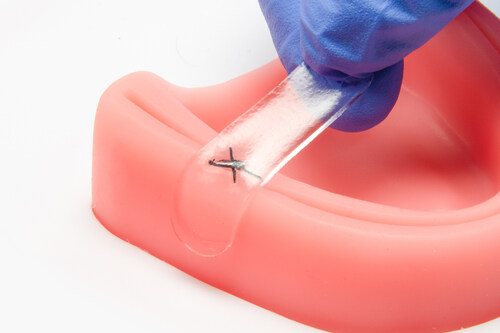
The achievement of ISO 38485:2016 certification underscores Amend Surgical’s commitment to safety, regulatory compliance, and operational excellence, confirming that the company maintains robust, auditable processes for the design, development, and manufacture of its NanoFUSE™ bone graft technologies. This milestone further strengthens the company’s manufacturing readiness as they advance their pipeline of next generation oral surgery solutions including the launch of Amend Tissue Tape™ and NanoFUSE™ for dental applications later this year.
“This is an important milestone for Amend,” said Robby Lane, CEO and Co-Founder, “Achieving ISO 13485 certification validates the strength of our quality systems and establishes a critical foundation for the next stage of our growth. As we move toward the anticipated regulatory clearance for Amend Tissue Tape™ in 2026, this certification ensures that we meet the highest global standards for safety and performance.”