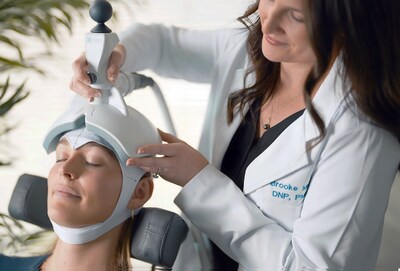
“We’ve never been more depressed yet more medicated. People deserve new options,” said Don Vaughn, Ph.D., neuroscientist and CEO of Ampa. “Ampa One was built to give clinicians a practical, portable, affordable tool that expands patient access to this lifesaving technology.”
In addition to its FDA-cleared device, the company is developing the Ampa One Day protocol, a groundbreaking approach that condenses a traditional multi-week TMS treatment into a single day. This innovation could define a new category of rapid-acting treatments for mental health.
“Ampa represents a revolution in TMS therapy,” says Tobias Marton, M.D., Ph.D., and CMO of Mindful Health Solutions. “Our clinical team has been impressed by its portability, intuitive design, and confirmed target engagement—features that redefine how and where TMS can be delivered. By enabling scalable next-generation protocols, Ampa is opening the door to broader access and better outcomes. Kudos to the Ampa team for bringing this transformative system to market.”