The St. Louis-based company also picked up Category B assignment from the Centers for Medicare & Medicaid Services (CMS). It said the two nods mark “a pivotal step” in its mission to address critical healthcare needs.
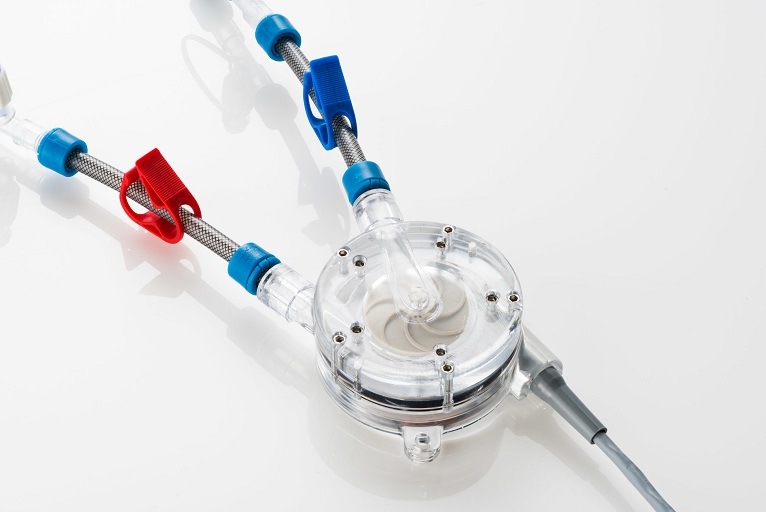
Amplifi Vascular designed its vein dilation system to dramatically enhance vein size and quality prior to arteriovenous fistula (AVF) creation. It aims to deliver more durable, reliable dialysis access and support the shift toward earlier, safer cannulation.
The system is comprised of a wearable, external blood pump with inflow and outflow catheters, plus a controller with a rechargeable battery. It rapidly dilates and preps the vein using well-understood hemodynamics. Removal occurs prior to AVF creation.