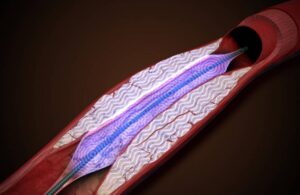
The POWER-PAD-II clinical study will evaluate the safety and efficacy of the company’s Pulse IVL system. Boston-based Amplitude designed it to treat patients with severely calcified peripheral arterial disease.
IVL is a hot space, highlighted by the recent closing of Johnson & Johnson’s $13.3 billion acquisition of Shockwave Medical. FastWave Medical, another figure in the space, recently reported positive study results for its own IVL technology.
Amplitude designed its IVL system to use pressure waves. Pulse IVL shatters intravascular calcium and expands the lesion to restore blood flow. The company aims to enroll 120 subjects in the trial, with follow-up lasting up to six months. A previous study of the technology already demonstrated benefits to patients with calcific femoropopliteal arteries, including reduced leg pain, increased blood flow and improved ability to walk.