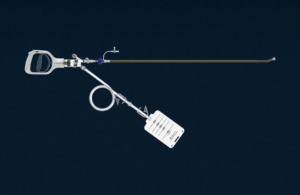
AlphaVac F18 is an emergent first-line device the company designed for the non-surgical removal of thromboemboli from the pulmonary arteries and for the treatment of pulmonary embolisms. It has an ergonomic handle, an 18Fr cannula with an 85-degree angle, an obturator and a waste bag assembly.
“The CE Mark represents a major step forward in enhancing patient care and safety for endovascular therapies in the EU, a market with a higher prevalence of PE when compared to the United States,” AngioDynamics’ Senior Vice President and General Manager of Endovascular Therapies Laura Piccinini said in a news release. “This designation allows us to broaden our reach and provide innovative solutions to more healthcare professionals treating patients diagnosed with PE – on an increasingly global scale.