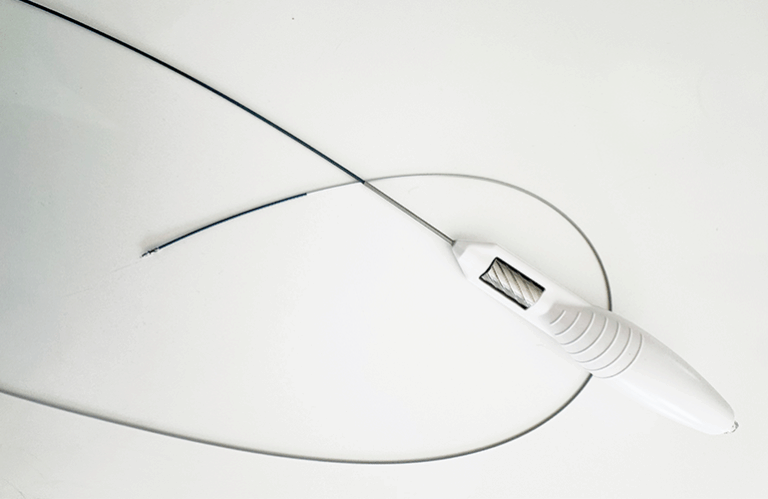
San Jose, California-based AngioSafe designed the device for wire-free intraluminal crossing of chronic total occlusions (CTOs) while simultaneously compressing plaque and recanalizing vessels. Santreva-ATK enables vessel preparation for imaging and treatment in a single step without an external power source.
The catheter is now commercially available in Europe and is expected to launch in the U.S. next month.
“AngioSafe was founded with a vision to restore blood flow safely, simply and reliably for patients with difficult-to-treat complex lesions by physicians with all experience levels,” said Sarvajna Dwivedi, co-founder, president and CEO of AngioSafe. “Our US and EU regulatory clearances, first real-world patient procedures, and emergence from stealth show that our vision is now becoming a reality. We look forward to advancing our mission and delivering lasting impact for patients, physicians, and the field of complex interventions, starting with Santreva-ATK as the first offering from our pipeline.”