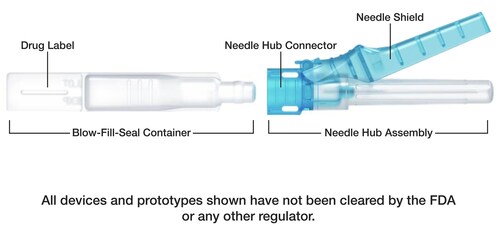
Central to today’s NDA submission of the drug Glycopyrrolate in the Apiject injection device is a drug delivery development platform that integrates two proven medical technologies: Blow-Fill-Seal (BFS) liquid packaging technology and precision injection molding of pen-style needle hubs. This combination enables the creation of a new category of prefilled drug delivery devices that are more scalable and affordable than traditional glass vials and prefilled syringes. The key for BFS is it is a continuous manufacturing process that operates at high-speed and relies on a single raw material that is available domestically.
Jay Walker, Co-Founder, Executive Chairman and CEO of Apiject, said, “Today’s submission is an exciting and significant step forward for Apiject. We have spent the last 5 years preparing BFS to play a central role in the future of drug delivery, because it meets the needs of the new challenges of today’s healthcare realities. Our work has involved extensive drug container and device design and development, building enhancements to the manufacturing process, inventing new equipment as well as extensive end-to-end testing. The global demand for medical injections continues to grow at a double-digit pace. We need new domestic capacity, more flexibility, lower costs, and we need new thinking.”