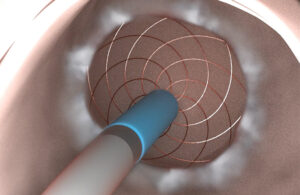
The IDE gives the device developer the green light to conduct a pilot clinical trial of its Proximal Intestinal Mucosal Ablation (PIMA) procedure using its proprietary radiofrequency vapor ablation (RFVA) system.
PIMA, a minimally invasive outpatient endoscopic procedure, delivers circumferential ablation to 50-70 cm of the proximal intestine. It uses a simple, through-the-endoscope RFVA catheter with no incisions or fluoroscopy required. It aims to replicate the metabolic benefits of gastric bypass while avoiding the invasiveness of the procedure.