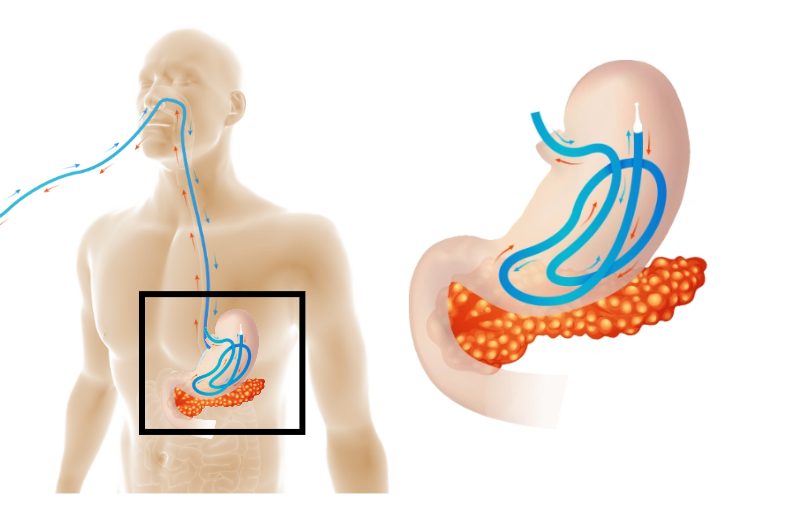
Arctx was granted FDA 510(k) clearance to market its flagship product, the Arctx Cool Catheter™ Set (“ACC,” “the Device”), for use in body temperature regulation. The ACC provides internal temperature management without requiring general anesthesia or vascular access. The Device includes a non-surgical, nasogastrically placed catheter within which either cold or warm water can be circulated in a closed circuit to provide patient cooling or warming. This marketing authorization represents another key milestone for Arctx Medical.
Arctx was also honored to have the results of its First-in-Human (FIH) clinical trial of the ACC for the treatment of Acute Pancreatitis (AP) selected for oral presentation as a Late Breaker during the 2025 American College of Gastroenterology annual conference. The Late Breaker results were presented by Dr. Walter Park of Stanford University, who remarked that “the initial results were encouraging and set the stage for CHILL-AP, the upcoming randomized, controlled pivotal trial of ACC therapy for AP.” Dr. Bernardo Goecke, the leading enroller in the FIH study, added that “The Cool Catheter performed reliably for patients and did not pose any safety concerns for us. Based on our positive experience with this therapy, we look forward to participating in the upcoming pivotal clinical trial.” Arctx was recently granted Investigational Device Exemption (IDE) approval by the FDA to conduct CHILL-AP at multiple leading US clinical investigation sites for patients presenting with AP.