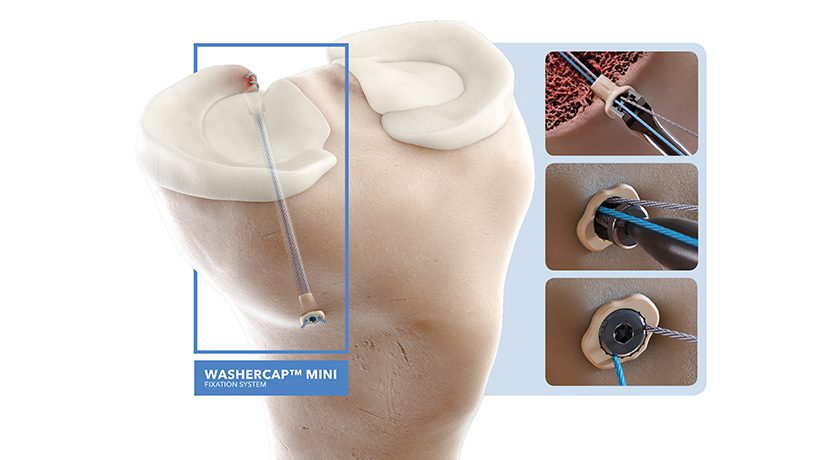
Current rotator cuff re-tear rates following surgery can range from 20%-40% in patients over 50.[1] Rotator cuff augmentation with biologic or biosynthetic grafts can mitigate this risk but can be technically demanding with current surgical techniques. The new SuperBall-RC product, based on Arcuro’s SuperBall technology platform successfully used in over 5,000 meniscus repairs, addresses this unmet need.
Philip Davidson, MD, Arcuro’s Medical Director, commented, “The SuperBall-RC has been designed to facilitate safe and easy fixation of rotator cuff augmentation grafts. The device has exceeded my expectations and offers a very attractive alternative to fixate augmentation patches and enhance healing.”
Jamal Rushdy, Arcuro’s CEO, added, “We are thrilled to have received this regulatory clearance, and I congratulate our product development and regulatory teams for their excellent work on this important milestone for the company which will help surgeons facilitate improved rotator cuff repair outcomes for their patients.”