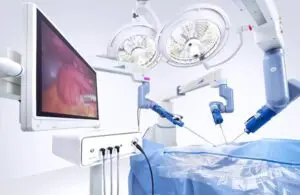
The Research Triangle Park, North Carolina-based company’s Senhance surgical system can now treat adult and pediatric urology patients.
Asensus originally designed Senhance for use in general laparoscopic and laparoscopic gynecological procedures. The company won FDA clearance for the system in 2017. Since then, it secured expanded indications, deals with Google and Nvidia, and hospital placements around the world.