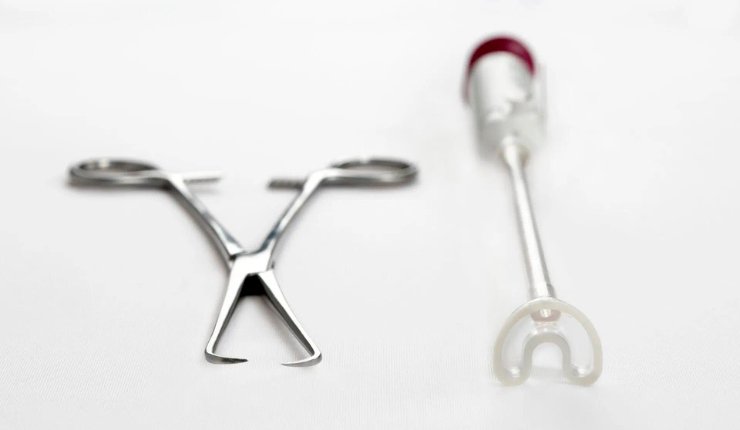
Aspivix has announced that its Carevix device has received approval from the Medicines & Healthcare products Regulatory Agency (MHRA) for use in the United Kingdom. Aspivix says this milestone marks a pivotal moment for the company and underscores its commitment to ‘modernising gynecology’ under its mission, ‘Gynecology. Now Modern.’
The company says that Carevix is designed to ‘redefine the standards’ of care in gynecology, offering a more comfortable and empowering experience for patients and healthcare providers alike for transcervical procedures in gynecology, oncology and fertility, including IUD/coil insertions.
“Receiving MHRA approval is not just an achievement for Aspivix but a victory for women’s healthcare globally,” said Mathieu Horras, CEO & Co-Founder of Aspivix. “This endorsement allows us to bring Carevix to the UK market, further expanding our reach and impact on women’s health. With existing FDA approval for the US and CE Mark for Europe, we are poised to make significant advancements in gynecological care.”