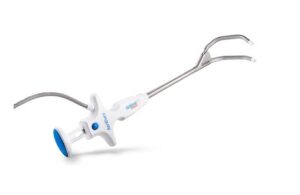
Following CE mark approval, the company says European surgeons already performed the first series of cases with EnCompass. The system received FDA 510(k) clearance and launched in the U.S. in 2022.
Mason, Ohio-based AtriCure designed EnCompass for the ablation of cardiac tissue during cardiac surgery to make concomitant surgical ablations more efficient. It allows physicians to perform a comprehensive epicardial ablation of the left atrium in just a few minutes