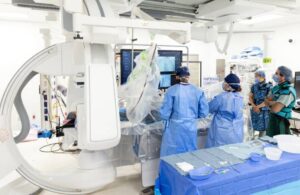
Remedy Robotics unveils remotely operable endovascular surgical robot
Remedy Robotics announced that it debuted the N1 system, a remotely operable endovascular surgical robotic system.

Remedy Robotics announced that it debuted the N1 system, a remotely operable endovascular surgical robotic system.
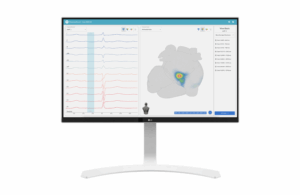
Vektor Medical today announced it received CE mark approval for its vMap artificial intelligence-powered arrhythmia mapping system.
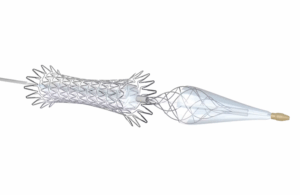
Medtronic (NYSE: MDT)+
today announced the full U.S. rollout of the Neuroguard IEP system, a carotid stent and embolic protection device.

Medtronic (NYSE: MDT)+
announced today that it kicked off the European launch of its VitalFlow one-system ECMO solution.
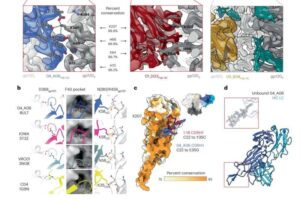
An international research team led by the University of Cologne has discovered an antibody that could advance the fight against HIV. The newly identified antibody 04_A06 proved to be particularly effective in laboratory tests. It was able to neutralize 98.5% of more than 300 different HIV strains, making it one of the broadest antibodies against HIV identified.
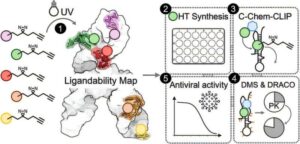
In a quest to develop new antiviral drugs for COVID-19 and other diseases, a collaboration led by scientists at The Wertheim UF Scripps Institute has identified a potential new drug against the virus that causes COVID-19.

GROVE CITY, Ohio, Oct. 6, 2025 /PRNewswire/ — Tosoh Bioscience, Inc., a market leader in clinical diagnostics is proud to announce that it has received US FDA 510(k) clearance for its next-generation system for HbA1c testing – the Tosoh Automated Glycohemoglobin Analyzer HLC®723-GR01 (hereafter “GR01”). The GR01 is intended for monitoring the long-term blood glucose control of individuals with diabetes, as an aid in the diagnosis of diabetes and to help identify those who may be at risk of developing the disease.
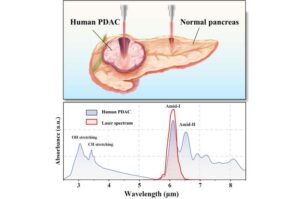
Researchers have developed a new laser-based technique that targets pancreatic ductal adenocarcinoma (PDAC) while leaving healthy tissue intact. PDAC is the most common type of pancreatic cancer and the third leading cause of death related to cancer.
A new way of diagnosing heavy bleeding after birth (postpartum hemorrhage or PPH) is more effective at identifying women in need of treatment than the current diagnostic method, suggests a meta-analysis published in The Lancet.
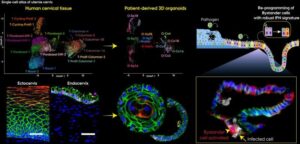
Cervical epithelial cells are far from passive bystanders in the body’s immune system. New research shows they actually play an active and highly coordinated role in detecting and fighting infections. That’s the conclusion of an international research team led by Associate Professor Cindrilla Chumduri from Aarhus University, published in the journal Science Advances.