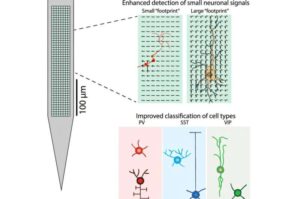
High-density brain probe reveals distinctive electrical patterns of cell types during behavior
Trying to document how single brain cells participate in networks that govern behavior is a daunting task. Brain probes called Neuropixels, which feature high-density silicon arrays, have enabled scientists to collect electrophysiological data of this nature from a variety of animals