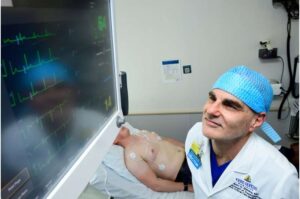
AI can predict complications from surgery better than doctors
A new artificial intelligence model found previously undetected signals in routine heart tests that strongly predict which patients will suffer potentially deadly complications after surgery. The model significantly outperformed risk scores currently relied upon by doctors.