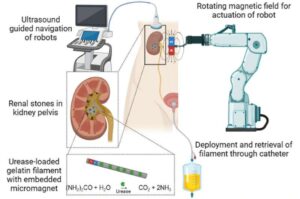
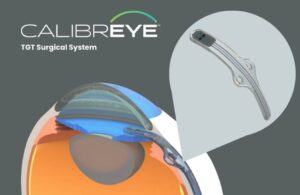
World’s first wireless OLED contact lens for retinal diagnostics developed
Electroretinography (ERG) is an ophthalmic diagnostic method used to determine whether the retina is functioning normally. It is widely employed for diagnosing hereditary retinal diseases or assessing retinal function decline.