
Modular Medical validates insulin patch pump cartridge for human-use production
Modular Medical (Nasdaq:MODD) announced today that it validated its insulin pump cartridge line for human-use production in the U.S.

Modular Medical (Nasdaq:MODD) announced today that it validated its insulin pump cartridge line for human-use production in the U.S.

In a small new study, a handheld saliva-sampling device successfully detected breast cancer 100% of the time, researchers said.
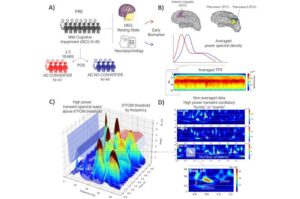
Using a custom-built tool to analyze the electrical activity from neurons, researchers at Brown University have identified a brain-based biomarker that could be used to predict whether mild cognitive impairment will develop into Alzheimer’s disease.
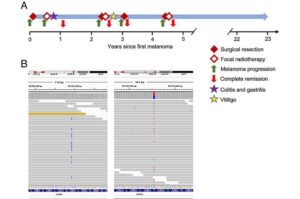
New research has uncovered a novel mechanism that may help explain why some people with cancer respond remarkably well to immunotherapy while others don’t.

University of North Carolina Lineberger Comprehensive Cancer Center researchers have developed a “two-in-one” molecule that can simultaneously turn off two notoriously difficult-to-target cancer-related genes, KRAS and MYC, as well as directly deliver drugs to tumors that express these genes. This advance holds special promise for treating cancers that have been historically challenging to treat.

https://medtechspectrum.com/news/40/24634/mirus-secures-ntap-approval-from-cms-for-breakthrough-europa-cervical-spine-system.html

SHENZHEN, China, Aug. 4, 2025 /PRNewswire/ — Pulsecare Medical, a pioneer in cardiovascular intervention technologies, announced that its innovative NxPFA™ nanosecond pulsed field ablation (ns-PFA) systemhas received marketing approval from China’s National Medical Products Administration (NMPA). As the world’s first third-generation ns-PFA system utilizing high-voltage nanosecond pulses for pulmonary vein isolation (PVI) in atrial fibrillation (AF) treatment, this breakthrough marks the dawn of the PFA 3.0 era and represents the innovative capabilities of Pulsecare Medical in cardiovascular multimodal therapy.

Stereotaxis (NYSE:STXS) announced today that it received FDA 510(k) clearance for its Magic Sweep navigation catheter.
Getinge announced today that European regulatory authorities reinstated the CE mark for its Cardiosave intra-aortic balloon pump.

In a study published in Advanced Materials, a research team developed an innovative bacterial cellulose (BC)-based hemostatic dressing that enables rapid and sustained bleeding control.