Brain imaging may identify patients likely to benefit from anxiety care app
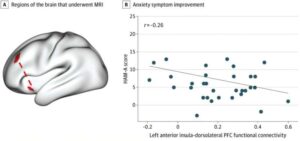
By understanding differences in how people’s brains are wired, clinicians may be able to predict who would benefit from a self-guided anxiety care app, according to a new analysis from a clinical trial led by Weill Cornell Medicine and NewYork-Presbyterian investigators.