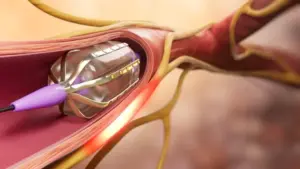
Autonomix Medical Secures New U.S. Patent for Advancing Nerve-Targeted, Minimally Invasive Therapies
Patent strengthens company’s IP portfolio as it expands innovative treatments for chronic pain, oncology, and other high-need conditions

Patent strengthens company’s IP portfolio as it expands innovative treatments for chronic pain, oncology, and other high-need conditions
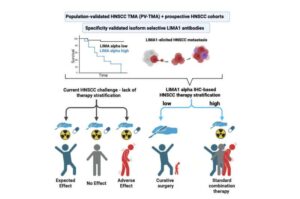
Researchers from the University of Turku and Turku University Hospital, Finland, led by Docent Sami Ventelä and Professor Jukka Westermarck, have developed a diagnostic tool that can revolutionize the treatment guidance of head and neck squamous cell carcinoma.
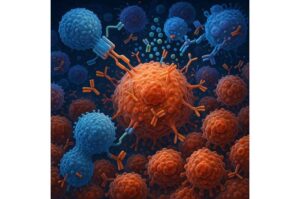
By sparking the immune system into action, radiation therapy makes certain tumors that resist immunotherapy susceptible to the treatment, leading to positive outcomes for patients, according to new research by investigators at the Johns Hopkins Kimmel Cancer Center Bloomberg~Kimmel Institute for Cancer Immunotherapy and the Netherlands Cancer Institute.
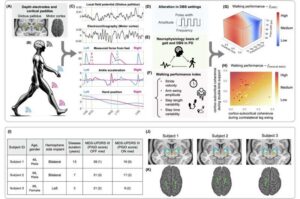
For patients with Parkinson’s disease, changes in their ability to walk can be dramatic. “Parkinson’s gait,” as it is often called, can include changes in step length and asymmetry between legs. This gait dysfunction reduces a person’s mobility, increases fall risk, and significantly impacts a patient’s quality of life.
An antibody treatment developed at Stanford Medicine successfully prepared patients for stem cell transplants without toxic busulfan chemotherapy or radiation, a Phase I clinical trial has shown.
The Global Neurodegeneration Proteomics Consortium (GNPC) has published a series of research papers detailing their efforts to identify patterns in neurodegenerative disease.
In a study conducted in Uganda and published in JAMA Surgery, researchers from Karolinska Institutet evaluated a new surgical method for treating groin hernias in women. The method could become an alternative in resource-limited settings where laparoscopic techniques are not generally available.
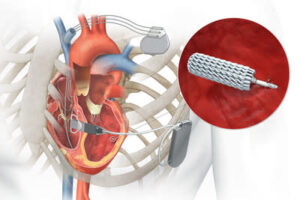
AUSTIN, Texas, July 21, 2025 /PRNewswire/ — Electrophysiologists at the Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center recently became the first in the nation to implant an FDA-approved novel leadless system that provides cardiac resynchronization therapy to patients with heart failure. Cardiac resynchronization therapy improves the timing of the heart’s contractions, helping to restore the normal rhythm of the heartbeat. The first procedure was recently performed by Robert Canby, M.D., cardiac electrophysiologist at TCAI.
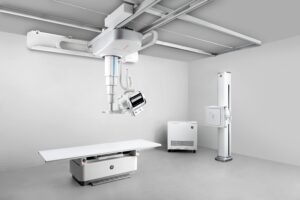
GE HealthCare (Nasdaq: GEHC)+
today announced the commercial launch of its advanced floor-mounted digital X-ray system.
Accurate removal of tumors is the most critical aspect of cancer surgery, yet it remains a significant challenge in clinical practice. In breast cancer, for example, the positive margin rate—where cancer cells remain at the surgical boundary—can reach up to 35%, often requiring reoperation and increasing the risk of recurrence.