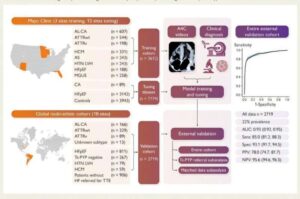
U.S. FDA Grants Breakthrough Device Designation to Artera’s AI-Powered Prostate Cancer Decision Support Tool
Innovative software platform leverages artificial intelligence to personalize therapy decisions and improve clinical outcomes for prostate cancer patients.