Real-time trial shows AI could speed cancer care
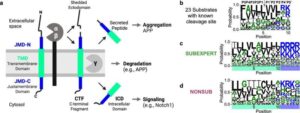
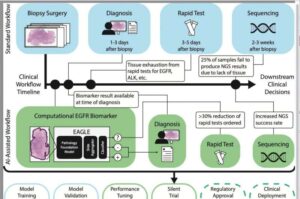
A new study by researchers at the Icahn School of Medicine at Mount Sinai, Memorial Sloan Kettering Cancer Center, and collaborators, suggests that artificial intelligence (AI) could significantly improve how doctors determine the best treatment for cancer patients—by enhancing how tumor samples are analyzed in the lab.