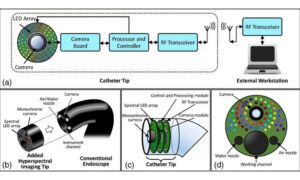
LED-based imaging system could transform cancer detection in endoscopy
Gastrointestinal cancers remain among the most common forms of cancer. While endoscopy has become a cornerstone of cancer screening and diagnosis over the past two decades, the procedure still misses approximately 8% to 11% of tumors, due to visibility limitations. Now, researchers have developed a prototype imaging system that could significantly improve doctors’ ability to detect cancerous tissue during endoscopic procedures.