Femasys Secures European Approval for FemBloc®
First-Ever Non-Surgical Permanent Birth Control System Gains CE Mark, Paving the Way for a New Era in Reproductive Health

First-Ever Non-Surgical Permanent Birth Control System Gains CE Mark, Paving the Way for a New Era in Reproductive Health

Next-Generation Ankle Replacement Platform Designed to Improve Surgical Precision and Patient Outcomes in Orthopaedic Care

Breakthrough Integrated Sacral Neuromodulation Device Approved to Treat Urinary Urge Incontinence with a Minimally Invasive, Patient-Centric Approach
Oxford researchers have developed programmable microcapsules to deliver vaccines in stages, potentially eliminating the need for booster shots and increasing immunization coverage in hard-to-reach communities.

A research team led by the University of the Basque Country has identified hundreds of molecular markers in saliva that could reveal the risk of a person developing major diseases such as cancer, cardiovascular diseases, diabetes and neurodegenerative diseases. Their results, published in npj Genomic Medicine, lay the foundation for the development of a powerful, non-invasive tool for early diagnosis and precision medicine.
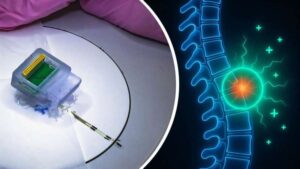
Researchers at Chalmers University of Technology in Sweden and the University of Auckland in New Zealand have developed a groundbreaking bioelectric implant that restores movement in rats after injuries to the spinal cord. This breakthrough offers new hope for an effective treatment for humans suffering from loss of sensation and function due to spinal cord injury.
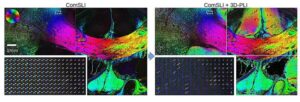
In order to understand the structure and functioning of the brain, neuroscientists need to study the complex, three-dimensional pathways and connections of nerve fibers. The intersection of multiple nerve fibers poses a particular challenge for neuroimaging.

Matthew Dean, Sales Engineer and Head of the Medical Business Unit at maxon, explores how Newrotex and maxon are developing an innovative approach to overcome the common challenges of traditional nerve repair.
Lilli Health, a company that coined the term Low Insulin Lifestyle [LILLI] created to help educate, treat and manage individuals with polycystic ovary syndrome (PCOS), has announced the launch of the Lilli App backed by decades of scientific research as the first-ever comprehensive platform focused on lowering insulin levels to reverse PCOS symptoms.

New artificial intelligence tools enhance early identification of high-risk polyps, improving colorectal cancer prevention and patient outcomes.