
Medline Secures FDA Clearance for REFLEX® HYBRID Nitinol Implants
New implants aim to enhance outcomes in foot and ankle surgeries through advanced material technology.

New implants aim to enhance outcomes in foot and ankle surgeries through advanced material technology.
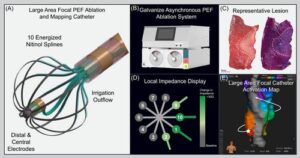
CardioFocus announced the completion of successful clinical cases with its QuickShot Nav large-area focal pulsed field ablation (PFA) catheter.

Precision Neuroscience announced today that it received FDA 510(k) clearance for its Layer 7 cortical interface for BCI technology
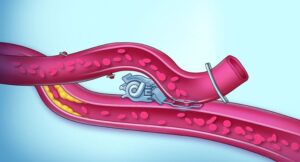
Adagio Medical (Nasdaq:ADGM) announced today that it received FDA breakthrough device designation for its vCLAS cryoablation system

SAN FRANCISCO, April 17, 2025 /PRNewswire/ — Solo Pace Incorporated, an emerging medical technology company, announced both FDA Clearance and First-In-Human use of their SoloPace Control System for temporary pacing in Transcatheter Aortic Valve Replacement (TAVR) procedures. With standardized workflows, the Control System is engineered to improve TAVR temporary pacing efficiency and reduce patient risks. Initial cases were completed this month at Scripps Clinic by Chief of Cardiology, Paul Teirstein, MD and Curtiss Stinis, MD.

Harvard’s Wyss Institute — a top medtech research institute in the U.S. — has received Stop Work Orders on three government contracts.
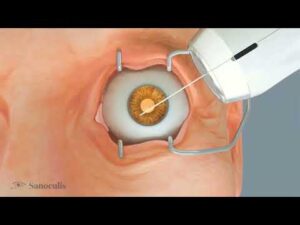
TEL AVIV, Israel, April 17, 2025 /PRNewswire/ — Sanoculis Ltd., a company specializing in ophthalmic medical device technologies, today announced that it has received CE Mark approval under the Medical Device Regulation (MDR) in the European Union (EU) for its MINT® (Minimally Invasive Nasal Trabeculostomy) product—an innovative, stent-free technology platform for the treatment of adult patients undergoing glaucoma angle surgery.

Scientists from the University of Bristol and the UK Atomic Energy Authority (UKAEA) have developed the world’s first carbon-14 diamond battery, capable of providing power for thousands of years.

Discover the groundbreaking Betavolt atomic battery, redefining energy with a 50-year charge cycle.
FREMONT, Calif., April 16, 2025 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced the successful first use of Maxx Orthopedics’ Freedom Total Knee implant utilizing the TMINI® Miniature Robotic System by Dr. David V. Cashen, a Joint Replacement specialist at Coastal Orthopedics Surgery Center in Bradenton, Florida.