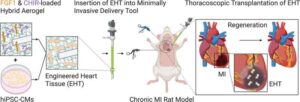
Researchers identify a new stem cell patch to gently heal damaged hearts
Mayo Clinic researchers have developed a pioneering method to mend damaged hearts without open-heart surgery, an advance that could one day transform the treatment of heart failure.