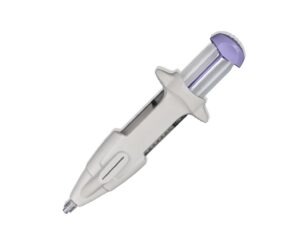
FDA clears Femasys’ FemChec diagnostic device
Femasys today announced it received FDA 510(k) clearance for its FemChec contrast-generating device that checks fallopian tubal status.

Femasys today announced it received FDA 510(k) clearance for its FemChec contrast-generating device that checks fallopian tubal status.
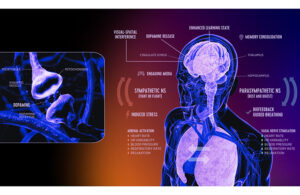
DeepWell Digital Therapeutics (DTx) has won FDA 510(k) clearance of technology to be used in immersive media like video games for stress reduction and as an adjunctive treatment for high blood pressure.
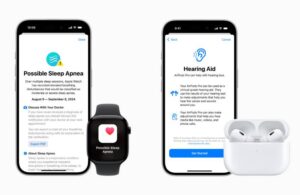
Apple today unveiled new sleep and hearing health features for its Apple Watch and AirPods Pro 2, expanding its health metrics offerings.

MGI Tech Co., Ltd. (“MGI”), a company committed to building core tools and technologies that drive innovation in life science, today announced the global rights to commercialize and distribute the new sequencing products CycloneSEQ-WT02* and CycloneSEQ-WY01*

The company says the minimally invasive system is set to ‘revolutionise’ care for patients as it allows for two separate devices to communicate for the first time.

For the first time, neuroscientists have developed a way to address debilitating PMS and menstrual pain symptoms without relying on drugs, hormones, or enduring severe side effects.
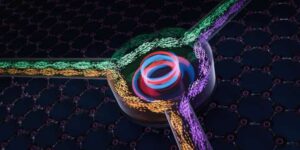
Researchers at ETH Zurich have managed to make sound waves travel only in one direction. In the future, this method could also be used in technical applications with electromagnetic waves.

The first crystal structure of an alternative DNA shape from the insulin gene has been revealed by a UCL-led research team.

Abbott (NYSE: ABT)+
today announced a major product launch, broadening the population that can utilize its continuous glucose monitoring (CGM) technology.

CENTER VALLEY, Pa., Sept. 5, 2024 /PRNewswire/ — Odin Medical Ltd., an Olympus Corporation company, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the first cloud-based Artificial Intelligence (AI) technology designed to assist gastroenterologists in detecting suspected colorectal polyps during colonoscopy procedures, the CADDIE™ computer-aided detection (CADe) device.