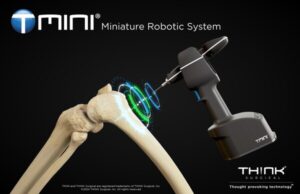
THINK Surgical Receives FDA 510(k) Clearance for Zimmer Biomet Persona Knee on TMINI® Miniature Robotic System
FREMONT, Calif., Sept. 5, 2024 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with the Persona® The Personalized Knee System® from Zimmer Biomet.