
FDA clears Orthofix Fitbone transport and lengthening system
Orthofix (Nasdaq: OFIX) today announced it received FDA 510(k) clearance for the Fitbone Transport and Lengthening System and reported the first implant of the device.

Orthofix (Nasdaq: OFIX) today announced it received FDA 510(k) clearance for the Fitbone Transport and Lengthening System and reported the first implant of the device.

Cordis announced today that the FDA granted premarket approval (PMA) for its Mynx Control venous vascular closure device (VCD).
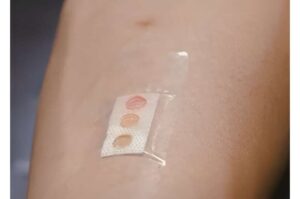
Scientists from Nanyang Technological University, Singapore (NTU Singapore) have developed a band-aid or plaster that measures body biomarkers that can indicate health or disease through sweat, paving the way for a new non-invasive and effective way for patients to monitor their health.

Pulse Biosciences (Nasdaq:PLSE) announced today that the FDA granted breakthrough device designation for its pulsed field ablation system. Shares of PLSE rose more than 20% in mid-morning trading on the back of this announcement.

Researchers at University of Tsukuba have pioneered a state-of-the-art wearable device capable of precisely and continuously measuring trace perspiration amounts. This innovative device is adept at monitoring perspiration levels during not only physical activity but also rest. Its potential applications extend beyond monitoring dehydration caused by exercise or heat exposure, encompassing broader domains such as daily health management and disease detection.
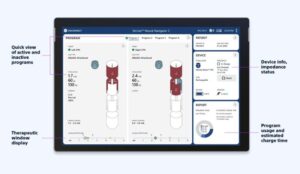
The Vercise Neural Navigator 5 with STIMVIEW XT technology is designed to streamline procedures for people living with neurological conditions according to the company.
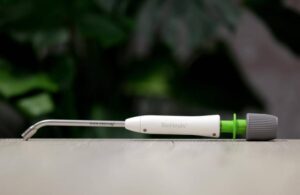
Signum Surgical announced today that the FDA granted de novo clearance for its BioHealx implant for treating anal fistula.

This tiny, biocompatible sensor may overcome one of the biggest hurdles that prevent the devices from being completely implanted.
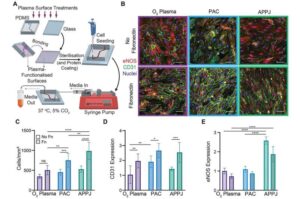
Researchers have developed a “blood vessel-on-a-chip” for heart disease with the potential to change the future of drug testing and development. The technology could also reduce our reliance on animal testing.
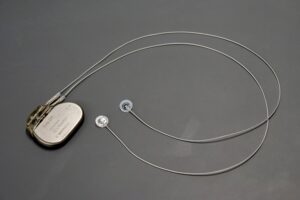
The minimally invasive, 3d-printable device offers safer application and removal, along with improved bioelectronic performance.